Commodity

Clay is sediments or rocks with greater than 67% clay-size (2 micrometers or less) or colloidal size particles. If the rock is unindurated, it is clay; if the rock is indurated, it is a claystone; if the rock is indurated and laminated, it is shale. If appreciable amount (34% or greater) of silt is present in an indurated clay-rich rock, the term mudstone is applicable.
Definition of Clay
The definition of clay is not as straightforward as looking up a definition in a dictionary. To the engineer, the term “clay” is used to describe cohesive particles having diameters less than 0.074 mm passing through U.S. standard sieve no. 200, in accordance with test method D422 of the American Society for Testing and Materials (ASTM). To a soil scientist, clay is any naturally occurring material composed primarily of fine-grained minerals. It generally is plastic at appropriate water content and will harden when dried or fired. A more academic definition often used by geologists actually gives three meanings for the term clay: 1) As a size term, different disciplines use different boundaries, but most place the boundary between silt and clay at either 2 or 4 micrometers; 2) Sediments or rocks with greater than 67% clay-size or colloidal particles; and 3) clay mineral. If the rock is unindurated it is clay; if the rock is indurated it is a claystone; if the rock is indurated and laminated it is shale. Industry refers to clays more in the context of the end use rather than the aggregate mineralogy.
H.A. Wheeler, who was the first to complete a comprehensive study on the clays of Missouri in 1896, defined clay as an earthy mineral that possesses more or less plasticity, and in which hydrous silicate of alumina is the dominating constituent. Ralph E. Grim, a noted clay mineralogist of the 20th century, believed in general the term “clay” implies a natural earthy, fine-grained material which develops plasticity when mixed with a limited amount of water. The material is the product of weathering, has formed by hydrothermal action, or has been deposited as sediment.
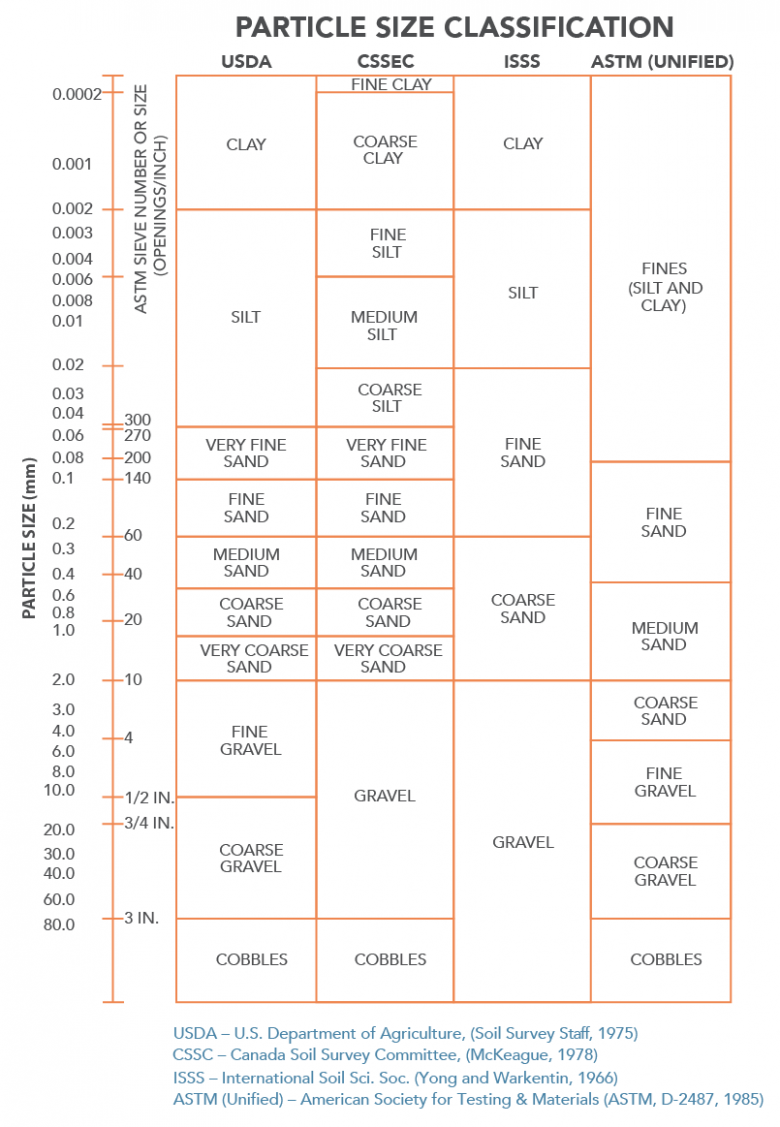
Sand and Gravel Particle Size
Different particle size classifications are based on different agency interpretations.
Industrial and Commercial Types of Clay
The following terms are the most commonly utilized by industry presently or historically to describe different types of clay deposits:
Refractory clay – Any clay showing a pyrometric cone equivalent of not less than cone 27. Before pyrometers (instruments that measure high temperatures) came into widespread use, pyrometric cones of materials that have a known melting point were used by kiln operators to measure and/or adjust the temperature of their kilns to the correct firing temperature dependent on the clay and product being produced.
Flint clay – A hard, smooth, nonplastic, usually kaolinitic claystone that breaks with conchoidal fracture and does not disperse in water. “Burley flint clay” is a claystone in which aluminum-rich minerals such as diaspore raise the Al2O3 content above that of kaolinite (~40%). Flint clays generally have an aggregate particle size of greater than 75 micrometers.
Burley clay – A clay containing burls (rounded inclusions of al-rich minerals); specifically a diaspore-bearing clay in Missouri (local name), usually averaging 45-65% alumina.
Diaspore clay – A high-alumina refractory clay consisting essentially of the mineral diaspore. It has been interpreted as a desilication (removable of silica) product of associated flint clay and other kaolinitic materials. Commercial diaspore of first-grade quality contains more than 68% alumina.
Fireclay – A plastic, kaolinitic claystone with sufficient Al2O3 to be refractory. It usually occurs as underclay. Flint clay should not be included in this category. Fireclays commonly occur as underclays. However, not all underclays are fireclays and thus not refractory.
Common clay – These clays are used to make heavy wares (e.g., bricks) that do not require a particularly pure type of clay deposit. These clays can come from a variety of sources such as loess, residual clays, alluvial clay and glacial clay.
Stoneware clay – A clay suitable for manufacture of stoneware (ceramic ware fired to a hard, dense condition with an absorption of less than 5%), used for items such as crocks, jugs and jars. It typically is high in kaolinite and possesses good plasticity, fusible minerals and long firing range.
Ball clay – A secondary clay, commonly characterized by the presence of organic matter, high plasticity, high dry strength, long vitrification range and light color when fired.
Bentonite - A commercial term applied to clay deposits containing smectite as the essential (main) mineral. This clay presents a very large total surface area, swells in water and is used chiefly to thicken oil well drilling mud.
Fuller’s earth – A) Naturally occurring clay or claylike material possessing a high absorptive capacity. It generally consists largely of hydrous phyllosilicates, smectite group minerals. Used as early as 5000 B.C. for whitening, degreasing or fulling (shrinkage, cleaning and thickening) of wool pelts and woolen fabric. Now extensively used as an absorbent in refining and decolorizing oils, fats and wine; it is a natural (but often modified bleaching agent). B) Any natural earthy material that will decolorize mineral or vegetable oils to a sufficient extent to be of economic importance. May be composed of attapulgite (palygorskite), smectite or kaolinite (Grim, 1953). C) In England, Fuller’s earth, known as creta fullonia, refers primarily to Cretaceous beds of calcium montmorillonite in southeast England that have been used for centuries (Robertson, 1986).
Kaolin – A soft relatively nonplastic but dispersible, usually white or nearly white claystone composed primarily of minerals of the kaolin group, principally kaolinite. It often contains a variable proportion of mica or quartz. Kaolin is white or nearly white on firing; a porcelain clay or natural (unwashed) china clay; used in the manufacture of ceramics, refractories and paper. Type locality: Kao-ling (meaning “high hill”), a hill in Jiang Xi Province, southeast China.
Potter’s clay – A plastic clay free from iron and devoid of fissility, suitable for modeling or making pottery or adapted for use on a potter’s wheel. It is white after burning.
Terra-cotta clay – Terra-cotta clays differ quite widely, but semifire clays or a mixture of these with a more impure clay or shale are mostly used. Buff-burning clays are preferred because of the hard body produced on burning. Desirable qualities in the terra-cotta clay include: dense burning character and strong bonding; low shrinkage and freedom from warping; and absence of soluble salts.
Gumbo – A term used locally in the United States for a clay soil that becomes sticky, impervious and plastic when wet.
China clay – A commercial term for low iron kaolin obtained from china-clay rock after washing, and suitable for the manufacturing of chinaware.
Porcelain clay – A clay suitable for use in the manufacture of porcelain; specifically kaolin.
Economic Importance
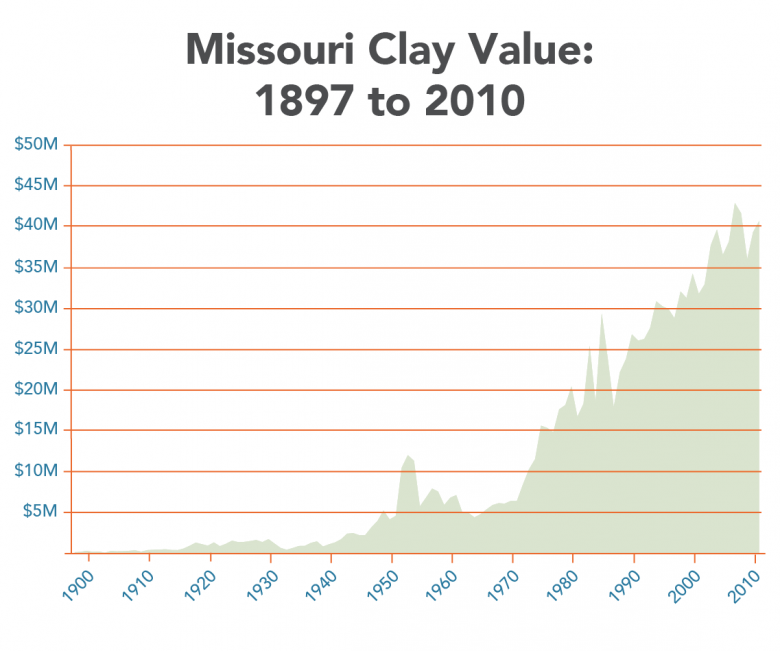
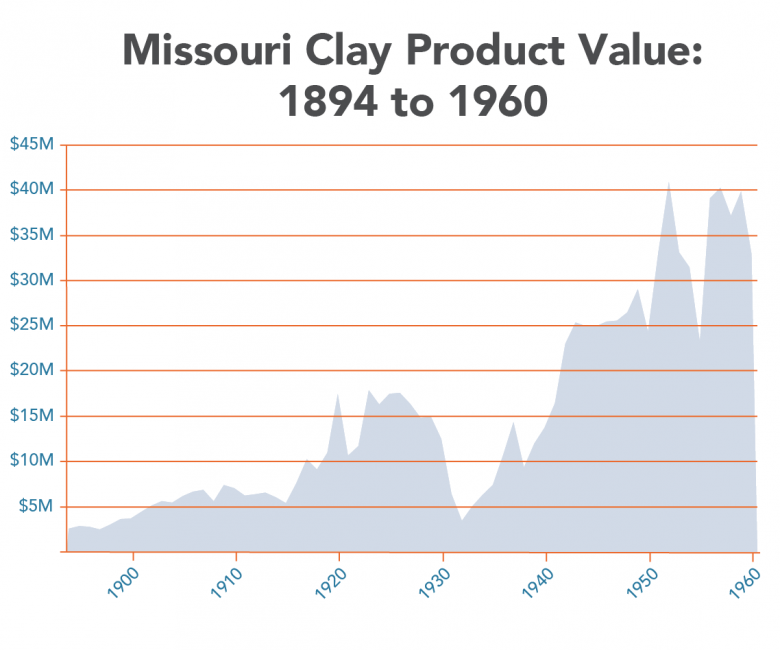
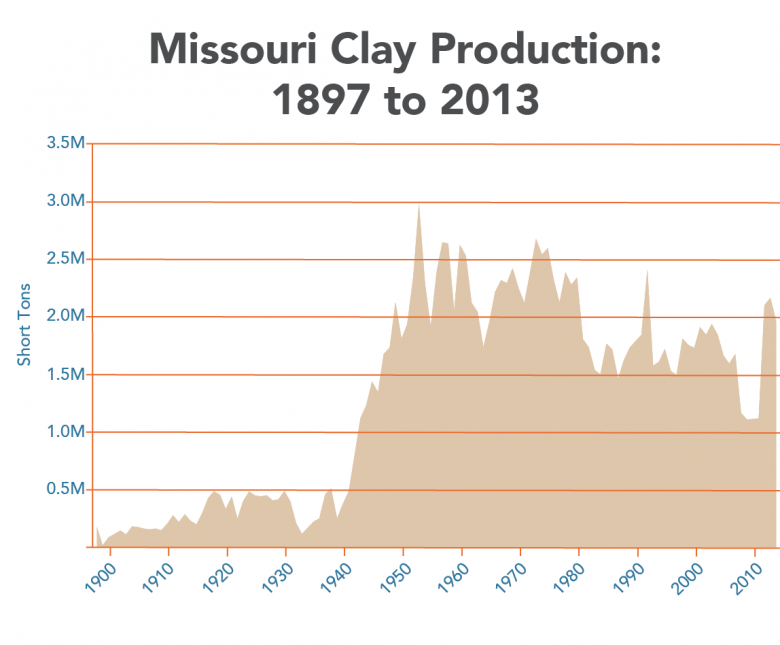
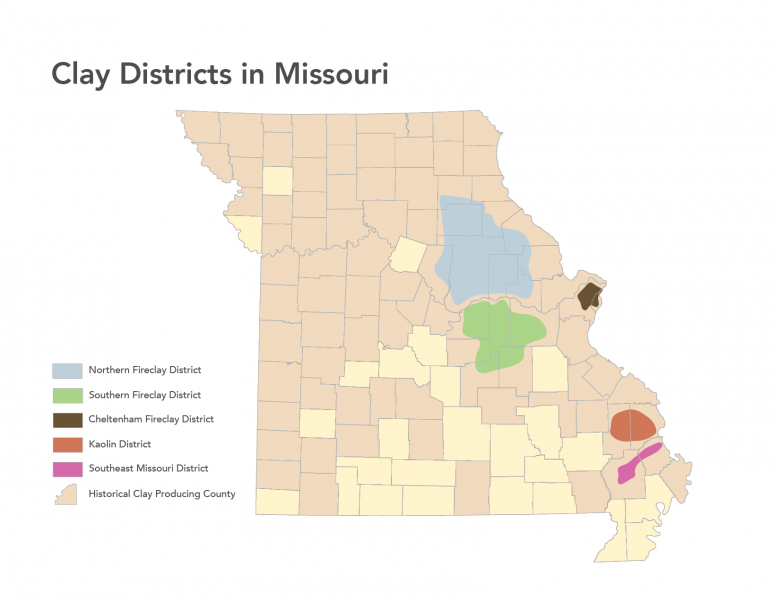
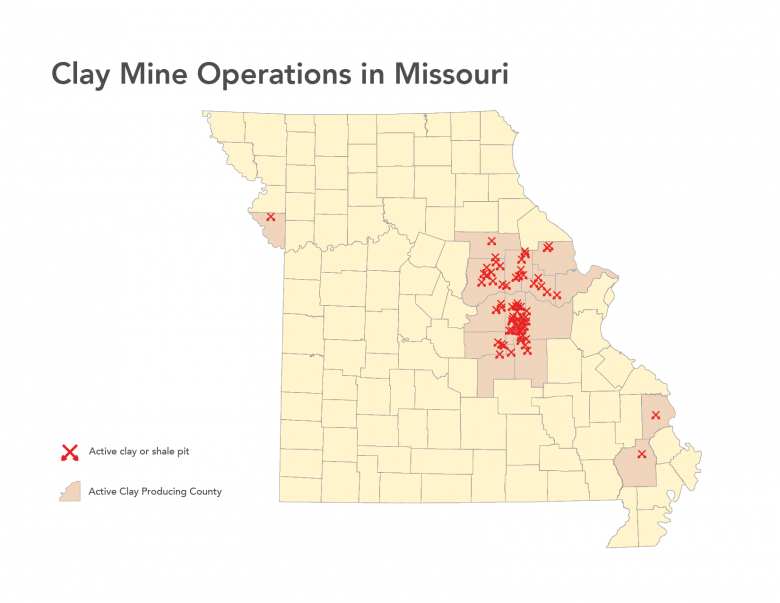
Clay and shales are necessary for modern society as we know it. Missouri is bountiful in common clay from loess deposits along the major rivers, alluvial clays found along many of the rivers and streams crossing the state, residual clays from 300 million years of weathering of igneous and sedimentary rocks to clays from north brought south by glaciation. Clay has been produced since Missouri joined the Union in 1821. By the 1850s, fire clay production in St. Louis was well established and today, fire clay production continues to contribute to the economy of the state. Common building brick was a large industry in Missouri during the first half of the 20th century. Missouri was the number one producer of refractory clay and Fuller’s earth (cat litter and other absorbents) in the United States in 2011 (latest available data). In 2011, Missouri ranked ninth in the U.S. for the production of common clay (bricks and cement). Currently, clay is being produced in 15 of Missouri’s 114 counties.
Uses of Clay and Shale

Clay has many different uses. Clay products are used in our everyday lives including in ways that are sometimes not so obvious. Ceramics pots are typically one of the first clay products that come to mind when thinking about clay products. This is justifiable as ceramics probably were the earliest clay product and one that is still important today. Clay is utilized for many building products such as bricks and adobe. It often is used as an aluminum source in the manufacture of Portland cement, a concrete ingredient. Our highways and large buildings depend on the strength concrete provides. The linings of smelting furnaces and boilers often are with special refractory clay bricks that can withstand the high temperatures. Oil and gas well drillers are dependent on clay as an additive to their drilling mud. Clay serves a vital role as filler for paper, plastics, rubber, caulking compound, paint and related products. Another major use of clay is as an absorbent such as kitty litter and to absorb oil. Similar clays have been used to remove discoloration of certain oil products.
Clays are also used in cosmetics not only as filler but as a coloring agent. In our pharmaceuticals, clay serves as filler and as an active ingredient in antidiarrheal medications. We even eat certain types of clay in our food when used as a filler.
While society and uses of clay have changed considerably through the years, the following are uses of clay and shale in Missouri, as reported by the producers at different times, showing the continuing importance of clay in everyday life.
Clay uses in Missouri – 1896
- Building brick
- Sewer pipe
- Firebrick
- Burnt ballast
- Paving brick
- Stoneware
- Terra-cotta
- Drain tile
- Roofing tile
- Flower pots
Clay uses in Missouri – 1915
- Firebrick
- Common brick
- Sewer pipe
- Front brick
- Vitrified brick, all uses
- Architectural terra-cotta
- Drain tile
- Fancy and ornamental brick
- Enameled brick
- Other tile
- Silica brick
- Stove lining
- Pottery
- Red earthenware
- Stoneware
- Rockingham ware

Clay uses in Missouri – 1948
- Firebrick
- Cement
- Sewer pipe
- Drain tile
- Chimney linings
- Various kinds of building bricks
- Flowerpots
- Abrasives
- Light weight expanded shale (Haydite)
Clay uses in Missouri – 1975
- Face brick
- Alum
- Firebrick
- High alumina firebrick
- Refractory mortar and cement
- Flue linings
- Flowerpots
- Grog
- Oil and grease absorbent
- Pet waste absorbent (kitty litter)
- Insecticides and fungicides filler
- Portland cement
- Sewer pipe
- Light weight expanded shale
- Structural concrete
- Concrete block
Recently reported (2010) uses of clay in Missouri
- Face brick
- Portland cement
- Light weight expanded shale
- Concrete block
- Structural concrete
- Highway surfacing
- Miscellaneous light aggregates
- Roofing granules
- Floor, wall and ceramic tile
- Firebrick
- Grog
- Pet waste absorbent (kitty litter)
Clay Minerals and Physical Properties
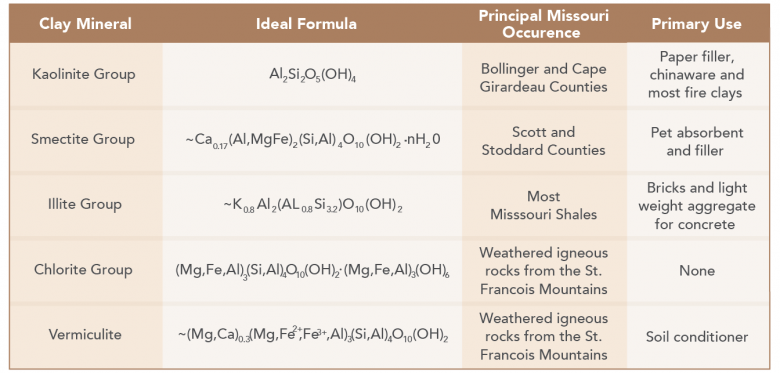
Clay Mineral – A colloidal size, crystalline, hydrous silicate with a crystal structure of the 3-layer type, kaolinite, or of the 2-layer type, montmorillonite, in which silicon and aluminum ions have tetrahedral coordination with respect to the oxygen, while aluminum, ferrous and ferric iron, magnesium, chromium, lithium, manganese and other ions have octahedral coordination with respect to oxygen or to hydroxyl groups (Figure 4). Exchangeable cations may be on the surfaces of the silicate layers in an amount determined by the excessive negative charge within the composite layer. These cations usually are calcium and sodium, but may also be potassium, magnesium, hydrogen, aluminum, etc. The most common clay minerals belong to the kaolinite, montmorillonite, attapulgite and illite (or hydromica) groups. Mixed layer clay minerals are either randomly or regularly interstratified intergrowths of two or more clay minerals.
Kaolinite – A) A common earthy white, grayish, yellowish, etc., triclinic clay mineral of the kaolin group: Al2Si2O5(OH)4. It is the characteristic mineral of most kaolins, and polymorphous with dickite and nacrite. Kaolinite consists of tetrahedrally coordinated silicon joined by oxygen shared with octahedrally coordinated aluminum (Figure 4); it also occurs as a disordered monoclinic variant. Kaolinite is a high-alumina clay mineral that does not appreciably expand under varying water content and does not exchange iron or magnesium. The mineral was formally known as kaolin. B) A name sometimes applied to the kaolin group of clay minerals, and formally applied to individual minerals of that group (such as dickite and nacrite).
Smectite – The accepted group name for 3-layer phyllosilicate clay minerals with layer charge between 0.2 and 0.6 per formula unit and which can take polar liquids into the interlayer space (Figure 4) causing them to swell perpendicular to the 001 surface (top surface). They have a high cation exchange capacity, about 110 cmolc kg-1 for soil smectites, and variable interlayering spacing. The smectite minerals are derived from the alteration of volcanic glass and form the weathering of primary silicates. They are the chief constituents of bentonites and Fuller’s earth and are common in soils, sedimentary rocks, and some mineral deposits.
Montmorillonite – A) A yellow, white or green monoclinic mineral that has the origin of the layer charge primarily in the octahedral sheet. An ideal formula is: (Na,Ca)0.3(Al.Mg)2Si4O10(OH)2.nH20. B) A name for a group of monoclinic dioctahedral micaceous minerals of analogous composition, but with (Na,Ca) replaced by Ca and (AL,Mg) replaced by Fe3+ or Cr.
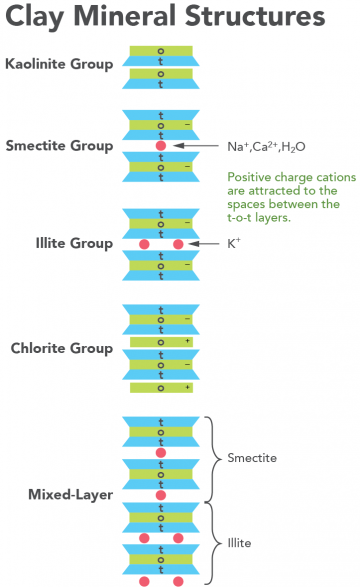
Illite – The term illite is used in two ways: in the general sense Grim et al (1937) introduced it as the 3-layer (Figure 4) muscovite-like monoclinic or rhombohedral mineral in the clay size fraction, that contains less potassium and more water than muscovite and yields a 10 Angstrom or one nanometer d(001) spacing from X-ray diffraction: (K,H3O)Al2(Si3Al)O10(H20,OH)2. Illite may contain as much as 5% of an interstratified component. This component is most commonly smectite, however, it may be vermiculite or chlorite. Srodon et al (1992) concluded that illite has a layer charge of -0.89. However, others find values as low as -0.70. In the treatment here, the term illitic material covers the general intention of Grim et al (1937), and the term illite should be used when referring to a specific mineral (Morre and Reynolds, 1996). In soil taxonomy, the presence of a 1nm X-ray diffraction peak and as much as 4% K2O is used to denote the presence of illite.
| 
|
Chlorite – A group of platy, monoclinic, usually greenish minerals with the general formula (R2+,R3+)4-6(Si,AL)4O10(OH,O)8. There are four subgroups of this 3-layer (Figure 4) layer clay mineral (the interlayer hydroxyl sheet is to be treated like other interlayer material.); 1) trioctahedral chlorite (the most common chlorites) i.e., both the octahedral sheet sandwiched between tetrahedral sheets and the interlayer sheet are trioctahedral; 2) dioctrahedral chlorite with both actahedral sheets dioctahedral, e.g., donbassite; 3) di,trioctahedral chlorite with the octahedral sheet in the 2:1 layer dioctahedral, but with the hydroxyl sheet dioctahedral. No examples of this mineral have been found. The common chlorites, the trioctahedral ones are named according to the dominant cation: Fe-rich is chamosite, Mg-rich is clinochore, Ni-rich is nimite, and Mm-rich is pennantite.
Vermicullite – A) A 3-layer layer monoclinic clay mineral distinguished from smectite on the basis of a higher layer charge, generally set at a greater than 0.6 per formula unit: Mg0.7(Mg,Fe,Al)6(Si,Al)8O20(OH)4.8H2O. B) A group name for sheet silicates with exchangeable cations.
Bentonite – A) A soft clay or greasy claystone composed largely of smectite formed by the chemical alteration of glassy volcanic ash in contact with water. It often contains accessory crystal grains that originally were phenocrysts in the parent rock. The rock commonly has the ability to absorb large quantities of water accompanied by a large increase in volume that can result in a thixotropic gel. The term “taylorite” was used by Knight (1898), after the owner of a quarry near Rock Springs in the Wyoming territory (see Taylor, 1987). It was later renamed “bentonite” after the Bent Formation (formerly Fort Benton Formation) in eastern Wyoming. B) A commercial term applied to clay deposits (especially bentonite) containing smectite as the essential mineral. This clay presents a very large total surface area, swells in water, and is used chiefly to thicken oil well drilling mud. C) Any clay composed dominantly of smectite clay mineral whose properties are dictated by this mineral (Grim and Guven, 1978, p.1).
Production History
Clay production in the area now encompassed by the state of Missouri started with the arrival of Native American people several thousand years ago. Native Americans utilized clay for various pottery, pigment and likely for medicinal purposes. By the mid 19th century, fireclay was being produced in the Cheltenham area of the City of St. Louis. At the end of the 19th century, St. Louis was the number one producer of heavy ware (bricks). The first half of the 20th century saw the largest growth of the refractory industry in Missouri. The discovery of high alumina fireclays in the southern fireclay district (Figure 5) was largely responsible. Missouri was the number one producer of refractory clay and Fuller’s earth (kitty litter and other absorbents) in the U.S. in 2011 (latest data available). In 2011, Missouri ranked ninth in the U.S. for the production of common clay (bricks and cement). Currently, clay is being produced in 15 of Missouri’s 114 counties.
Common Terms Used in the Clay Industry
- Argillaceous – A) Pertaining to, largely composed of, or containing clay-size particles or clay minerals, such as an “argillaceous ore” in which the gangue is mainly clay; especially said of a sediment (such as marl) or a sedimentary rock (such as shale) containing an appreciable amount of clay. B) Said of the peculiar odor of argillaceous rock when breathed upon. C) Pertaining to argillite.
- Burl – A nodule in fireclay. It may have a high content of alumina, iron oxide or siderite.
- Chinaware – An expression describing porcelain, particularly porcelain tableware.
- Fireclay mineral – A disordered variety of kaolinite.
- Fissility – A general term for the property possessed by some rocks of splitting easily into thin layers along closely spaced, roughly planar, and approximately parallel surfaces, such as bedding planes in shale or cleavage planes in deformed rocks; its presence distinguishes shale form mudstone. The term includes such phenomena as bedding fissility and fracture or spaced cleavage. Etymol: Latin “fissilis,” - “that which can be cleft of split.”
- Glass pot – A fireclay and grog or sillimanite crucible used in melting glass.
- Grog – Burned clay. It is used to reduce the shrinkage of plastic clays and to give additional porosity. Grog enables refractory goods to withstand sudden changes in temperature. It often is obtained by grinding old firebricks, or by burning a high-grade fire clay in a shaft kiln out of contact with fuel and grinding the product to a coarse powder and removing the dust.
- Heavy Clay Products – Products typically made from “common clay” include building brick, drain tile, sewer pipe, etc.
- Plasticity – The property of a material being deformed under the application of pressure, with the deformed shape being retained when the deforming pressure is removed.
- Refractory – A) Said of an ore from which it is difficult or expensive to recover its valuable constituents. B) Exceptionally resistant to heat.
- Retort – A) A vessel used for the distillation of volatile materials, as in the separation of some metals and the destructive distillation of coal B) A long semicylinder, now usually of fireclay or silica, used for the manufacture of coal gas.
- Rockingham ware – The term originally referred to the ornate porcelain made at a pottery at Swinton, Yorkshire, England. Ware with a brown manganese glaze also was produced and it is this type of glaze, which in the United Kingdom, is usually applied to teapots made from red clay, to which the term Rockingham ware now refers.
- Slip – A) A mixture of fine clay and water having the consistency of cream, used in the casting process for the decoration of ceramic ware, or as a cement for handles and other applied parts. B) Enamel or glaze, powdered and suspended in water and ready for application. C) A suspension of ceramic material in a liquid. Also sometimes called slurry.
- Terra-cotta – A burned clay product widely used for ornamental work on the exterior of buildings.
- Vitrification – Formation of glass.
- Whiteware – A) As applied in ceramics, refers to claywares made from clay bodies which fire to a white color. B) Ware with a white- or ivory-firing body.
References and Additional Reading
Bishop, O.M., 1949, The mineral industry of Missouri in 1946 and 1947 with total production summarized, Missouri Geological Survey and Water Resources, Information Circular No. 4, 93 p.
Buehler, H.A., 1917, Biennial report of the state geologist to the 49th general assembly, Missouri Bureau of Geology and Mines, Rolla, MO, 75 p.
Lefond, S.J. (editor), 1983, Industrial mineral and rocks, Volume 1, American Institute of Mining, Metallurgical, and Petroleum Engineers, Baltimore, Maryland, 753 p.
Neuendorf, K.K., Mehl, Jr., James P., and Jackson, J.A. (Editors), 2011 Glossary of geology, American Geosciences Institute, Alexandria, VA, 783p.
Thornberry, M.H., 1925, A treatise on Missouri clays, School of Mines and Metallurgy, University of Missouri, Technical Series, vol. 8, no. 2, 69 p.
Thrush, Paul W. (compiler), 1968, A dictionary of mining, mineral, and related terms, U.S. Bureau of Mines, Washington D.C., 1269 p.
Wheeler, H.A., 1896, Clay deposits, Missouri Geological Survey, vol. 9, 2nd series, Jefferson City, 622 p.
Search the Missouri Geology Bibliography
Visit the department’s Ed Clark Museum of Missouri Geology, where you will find clay and shale on display.