Missouri Geological Survey Director: Carey Bridges, RG
Commodity
Zinc is a brittle, whitish metal used in a wide range of industries, most familiarly as a galvanizing material for steel. Known and used for millennia, it was named “zinc” in the early 1500s by Paracelsus, a Swiss physician and philosopher. The name was derived from “zinke,” a German word for “fork tooth,” referring to the form zinc often took when smelted. It was not formally identified as an element until 1746.

Sphalerite (ZnS) is the primary zinc ore in Missouri. Sphalerite currently is produced from multiple mines in the southeastern part of the state, where it is associated with lead, copper and silver. Past mining was extensive in the southwestern part of the state. Early zinc mining in Missouri also included the minerals smithsonite (ZnCO3), hemimorphite (Zn4Si2O7(OH)2·H2O), and hydrozincite (Zn5(CO3)2(OH)6).
Sphalerite is also a source for silver in southeast Missouri. While silver is not part of the chemical formula of sphalerite, the silver atom fits in gaps in the crystal structure of sphalerite and is recovered when zinc ore is smelted. Other elements associated with sphalerite mined in southwest Missouri include germanium, cadmium, gallium and indium.
Economic Importance
Missouri has been a significant zinc producer since the mid-1860s. Zinc mining was a major driver for development, especially in the southwestern portion of the state. Zinc dominated production in the Tri-State District from 1872 until closure of the last mine in Missouri in 1957. Jasper County, center of the Missouri portion of the Tri-State District, almost tripled in population from 1880 to 1900 due to the mining boom, while neighboring Newton County nearly doubled from 1870 to 1900. Mining in this district ceased in Missouri in 1957, although production had already dropped to less than half of previous levels after 1917. Smaller areas in Lawrence, Greene, Franklin and Jefferson counties also led to expanding populations and development. Zinc production was closely tied to lead mining in St. Francois and Madison counties, with lead as the dominant ore.
Missouri currently is the second largest producer of zinc in the U.S., with production from the Viburnum Trend. With the exception of two years, Missouri led the U.S. in production from 1874 to 1918. Zinc production in Missouri totals nearly 6.5 million tons. Based on 2018 prices, Missouri zinc has produced nearly $19 billion dollars of value throughout its history.
Zinc Ore Minerals in Missouri
Sphalerite
- Chemical composition: ZnS.
- Color and Luster: color varies from yellow to light to dark brown, red-brown, red, black, and colorless, and is less commonly light blue or green. It has a resinous to adamantine luster.
- Hardness: 3.5 to 4.
- Cleavage: 1 perfect.
- Specific gravity: 3.9 to 4.1.
- Crystal system: Isometric crystal system
- Named from the Greek "sphaleros," meaning "treacherous," due to the difficulty in telling darker varieties from other ore minerals.
Smithsonite
- Chemical composition: ZnCO3
- Color and Luster: color is highly variable, ranging from white to gray, yellow, green, blue, pink, purple, bluish gray, or brown. Luster is vitreous or pearly.
- Hardness: 4 to 4.5.
- Cleavage: 1 very good.
- Specific Gravity: 4.4.
- Crystal system: Trigonal
- Smithsonite was named for James Smithson, founding donor of the Smithsonian Institution.
Hemimorphite
- Chemical composition: Zn4Si2O7(OH)2·H2O
- Color and Luster: color varies from colorless to white, pale blue, pale green, gray, or brown. Luster varies greatly, from vitreous to greasy, silky, pearly, or sub-adamantine.
- Hardness: 4.5 to 5.
- Cleavage: 3 cleavages – 1 perfect, 1 poor, 1 rare.
- Specific Gravity: 3.5.
- Crystal system: Orthorhombic
- Hemimorphite was named for its crystal shape.
Hydrozincite
- Chemical composition: Zn5(CO3)2(OH)6
- Color and Luster: color varies from white to gray, pale pink, pale yellow, or brown. Luster varies from silky to pearly, dull, or earthy.
- Hardness: 2 to 2.5.
- Cleavage: 1 perfect.
- Specific Gravity: 3.5 to 4.
- Crystal system: Monoclinic
- Hydrozincite was named for its chemical composition.
Common Names
Sphalerite is also known as blende, zinc blend and jack. It has also been called ruby jack, black jack, rosin jack and steel jack, based on its color. "Calamine" was often used to describe a naturally occurring mix of smithsonite, hemimorphite and hydrozincite, and was common usage in southwest Missouri.
Primary Uses
The majority of zinc is used to make galvanized steel. It is also used for zinc-based alloys, in brass and bronze, die casting, fertilizers and fungicides, and as chemical compounds in multiple industries, including the pharmaceutical and agricultural industries. It is a critical element for healthy development of humans, plants and animals, an ingredient in multiple medicines and a major component of sunscreen. The U.S. 1-cent coin, known as a “copper penny” actually only contains 2.5% copper as plating; the remaining 97.5% is zinc.
Past Uses
An early use of zinc was as an alloy with copper to make brass as early as 2,500 years ago. It was also used in early coins, medicine and leather dressing. Zinc as a metal was unknown until first smelted in the 13th century. The most unusual historic use for sphalerite may have been in the Joplin region where, during the U.S. Civil War, the unusable (at that time) sphalerite ore was used to build a stockade against raiders.
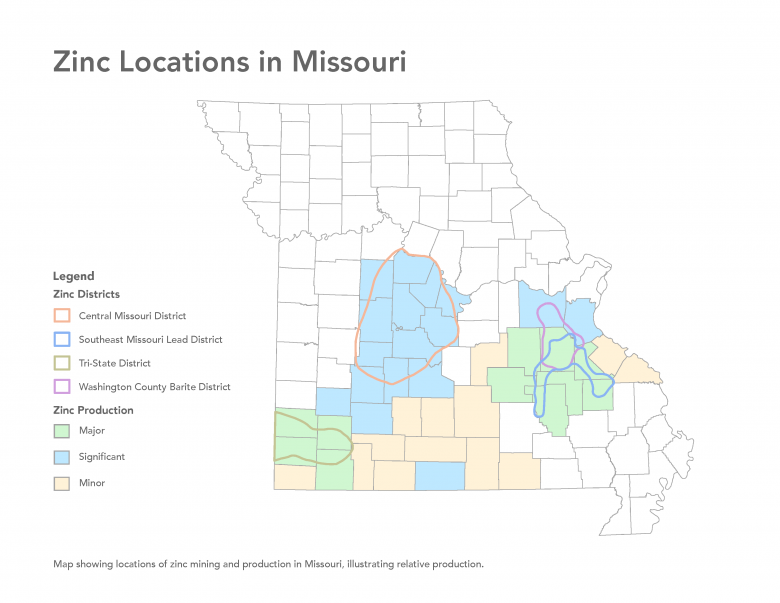
Principal Locations of Zinc in Missouri
The majority of zinc mining occurred in several districts in the southern part of the state. The Tri-State District and Southeast Missouri Lead District are considered world-class mining districts. Minor zinc mineralization is found throughout large parts of Missouri.
Tri-State District
- So-named as it covered portions of southwest Missouri, northeast Oklahoma and southeast Kansas.
- Tri-State produced zinc and lead in Missouri from the 1830s to 1957, and enabled Missouri to lead the US in zinc production from the mid-1800s through the 1910s.
- The district also produced cadmium and indium during World War II and contained germanium and gallium.
- Zinc mining strongly influenced settlement and development of several areas of Missouri, most strongly in the southwestern counties of Jasper, Newton and Lawrence.
Southeast Missouri Lead District
- Comprises several subdistricts.
- The Viburnum Trend (1955 to present) continues to produce zinc along with lead, copper and silver. It is located in Crawford, Dent, Iron, Reynolds, Shannon and Washington counties.
- Mine LaMotte-Fredericktown (1720-1961), located in Madison and St. Francois counties produced zinc along with lead, copper, cobalt, and nickel. The Old Lead Belt (1700s to 1972), located in St. Francois County, produced lesser quantities of zinc.
- This district has ensured Missouri's position as the second largest producer of zinc in the U.S. for decades.
Several smaller districts also historically produced zinc including: the Central Missouri District and Washington County District, where zinc was produced with barite and lead, and the Aurora District (Lawrence County), Valle Mines (Jefferson County), and Greene County, where lead was produced along with zinc. Zinc mining also occurred throughout multiple other counties in southern Missouri, most notably Barry, Christian, Dade, Douglas, Franklin, Ozark, Webster and Wright, among others. Small-scale zinc mining also occurred in multiple other counties in southern Missouri.
Geologic Occurrence

Zinc mineralization in Missouri is found in Mississippi Valley-type deposits; ore deposits with characteristic mineralization that formed in limestone and dolomite in specific geologic settings.
Sphalerite and other zinc-bearing minerals are primarily found in Cambrian- and Ordovician-age rocks in southeastern Missouri, in Ordovician-age rocks in the Central Missouri District, and in Mississippian-age rocks in the Tri-State District. Smaller mining areas had ore hosted by Cambrian- through Mississippian-age rocks. Sphalerite and other zinc-bearing minerals are found as minor occurrences in most geologic age rocks in Missouri.
The ores are found in limestone and dolomite, and as residual ores in weathered ore-hosting rocks. Ores also are common in jasperoid, a rock similar to chert, in the Tri-State District. Ore minerals occur as crystals in open spaces and fractures, and as fine grains of ore disseminated through and replacing the host rock. Disseminated ores comprise the majority of currently economic zinc ores in Missouri. The Joplin area was especially noted for the large crystals of sphalerite and other minerals found in open cavities during mining.
Despite these similarities, there are differences in the occurrence of zinc ores in the different districts.
In the Southeast Missouri Lead District, zinc ores are generally disseminated or found as open space fill. They appear to have been concentrated by several mechanisms including: limestone-dolomite boundaries, solution-collapse breccias, faulting and fracturing of the host rock, pinchouts of overlying shales, or changes in host rocks related to buried Precambrian topography. An individual ore deposit will often exhibit several of these mechanisms acting in combination.
Mineralization in the Tri-State District is strongly controlled by folding and fracturing of the host rocks. Ores occurred in multiple forms, each with names well-ensconced in historic literature. "Circle" deposits are associated with sinkholes, and hosted the large, well-known surface mines of Oronogo Circle and Sucker Flats. "Runs" hosted the greatest quantities of ore and are linear bodies that follow fracturing or shear zones, often marked by extensive replacement of the host rock by silica or jasperoid. "Sheet ground" deposits have mineralization that followed bedding planes or other horizontal features of the rocks.

Mining
Ores have been mined at the surface in the past and currently are mined at depths as great as 1,400 feet in the mines in the Viburnum Trend. Ores are blasted, crushed, transported to the surface, crushed further, and run through flotation cells to separate the zinc ore from the dolomite or limestone and from other ore and non-ore minerals. Ores are then smelted to generate zinc metal.
Early zinc mining throughout the state was of shallow or near surface ores, with limited underground workings. The Tri-State District experienced the most extensive mining. Early Tri-State mining was done on a lease and royalty system with landowners dividing their tract of land into leasing claims as small as 100 square feet. This leasing method remained prominent through the 1890s and persisted into the 1930s. Early mining of shallow ores lead to numerous shafts dug in close proximity to one another. Improvements in groundwater pumping technology in the late 1800s lead to development of larger underground mines.
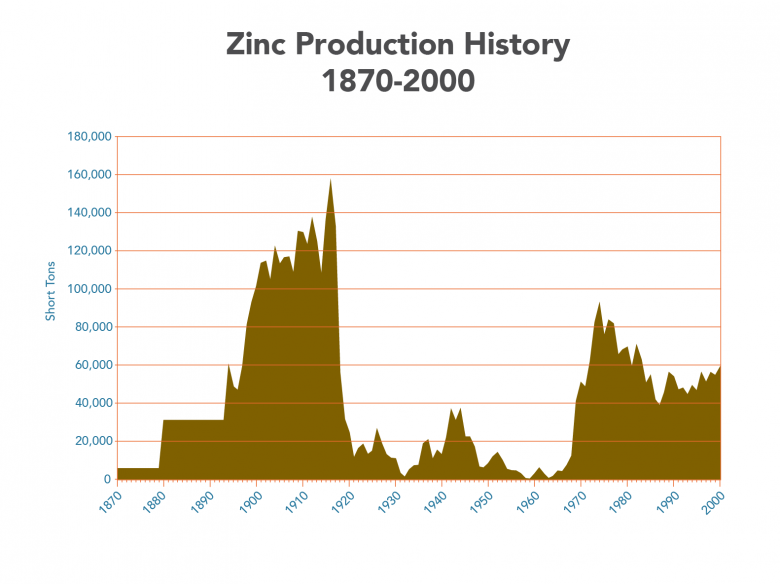
Production History
Zinc production in Missouri from 1870-2000; data for years later than 2000 are not available. Values pre-1894 are averaged from lump sums. The drop near 1920 marks a decrease in production in the Tri-State region; the increase near 1970 marks increased production from the Viburnum Trend.
Additional Reading
Search the Missouri Geology Bibliography.
Visit the department’s Ed Clark Museum of Missouri Geology, where you will find barite on display.
Nothing in this document may be used to implement any enforcement action or levy any penalty unless promulgated by rule under chapter 536 or authorized by statute.
For more information
Geological Survey Program
Missouri Geological Survey
P.O. Box 250
Rolla, MO 65402-0250
United States